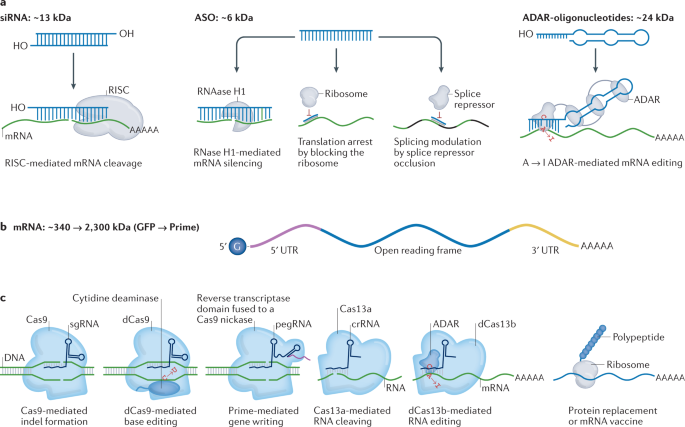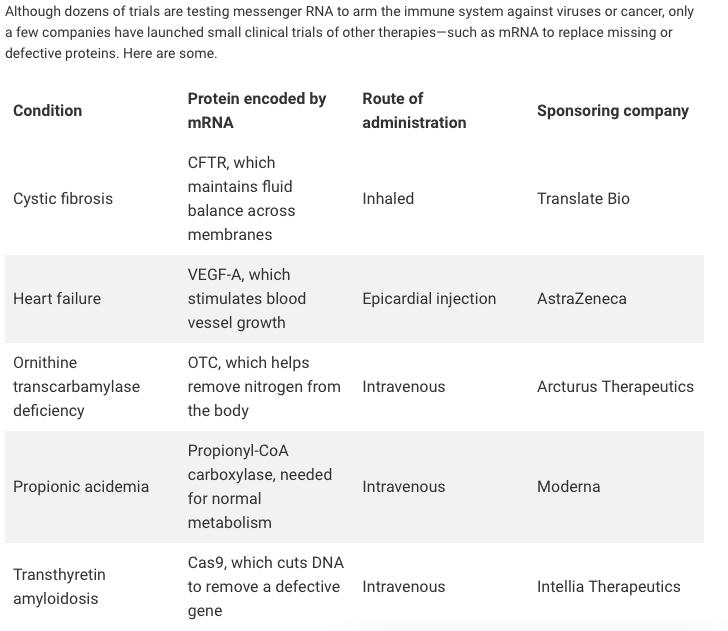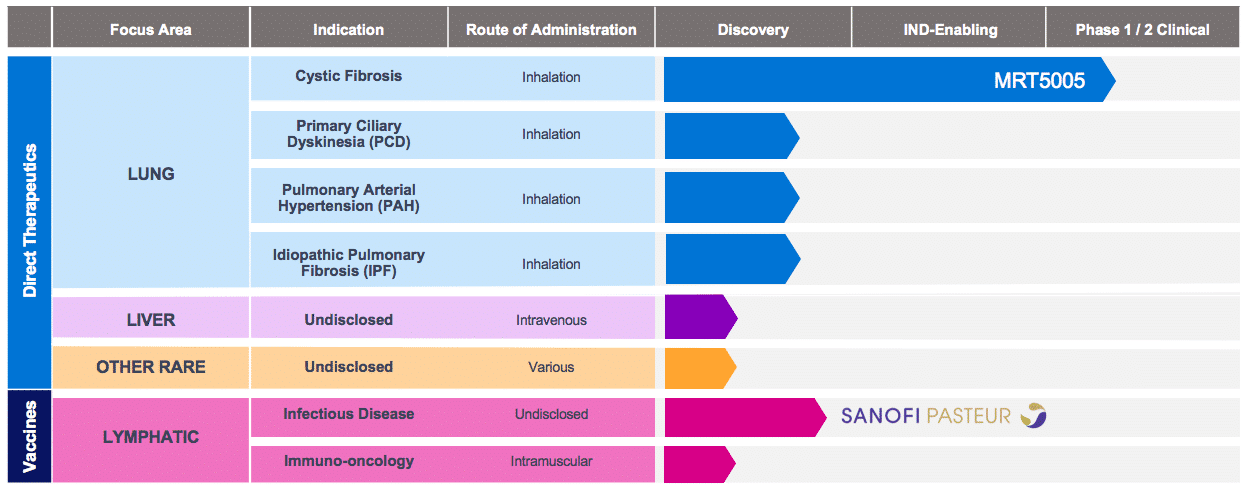
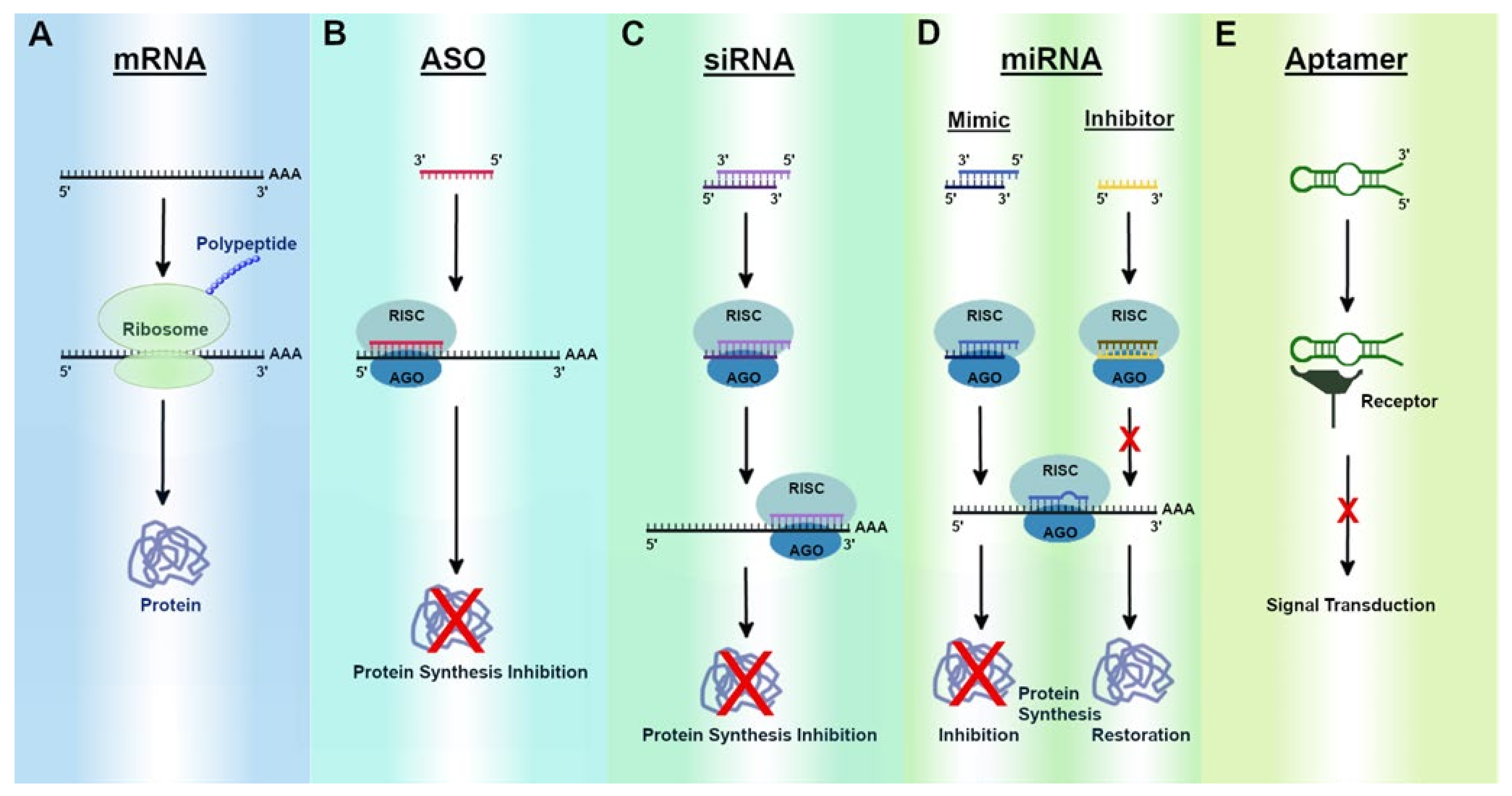
Translate Bio Should Be On Your Radar With Upcoming Presentation And CF Data In 2020 (NASDAQ:SNY) | Seeking Alpha

Kate Eveling on Twitter: "🫁 BiomX are developing BX004 Phage therapy, viruses that target & kill specific bacteria, in this case chronic Pseudomonas aeruginosa. They are advancing into Phase 1b/2a trial 🫁Beyond

Translate Bio Announces Interim Results from Phase 1/2 Clinical Trial of MRT5005 in Patients with Cystic Fibrosis – OCTOPUSYX BIOCONSULTING

Translate Bio Announces FDA Clearance to Proceed with Phase 1/2 Clinical Trial in Patients with Cystic Fibrosis (CF) | Business Wire











![Top 10 Cystic Fibrosis Clinical Trials [2022 Studies] | Power Top 10 Cystic Fibrosis Clinical Trials [2022 Studies] | Power](https://pwr-og-img.withpower.com/Cystic%20Fibrosis%20research%20studies%20recruiting%20patients%20in%202022%20need%20your%20help.%20Receive%20premium%20care%20&%20cutting%20edge%20treatments%20by%20enrolling%20in%20cystic%20fibrosis%20clinical%20trials%20today..png?md=1)