Frontiers | Current Treatment Options and Therapeutic Insights for Gastrointestinal Dysmotility and Functional Gastrointestinal Disorders | Pharmacology

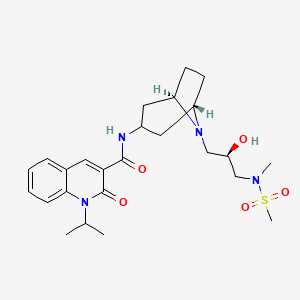
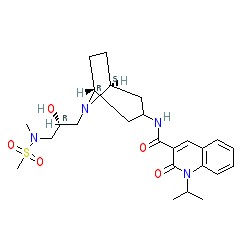
Clinical trial: the efficacy and tolerability of velusetrag, a selective 5‐HT4 agonist with high intrinsic activity, in chronic idiopathic constipation – a 4‐week, randomized, double‐blind, placebo‐controlled, dose–response study - Goldberg - 2010 -

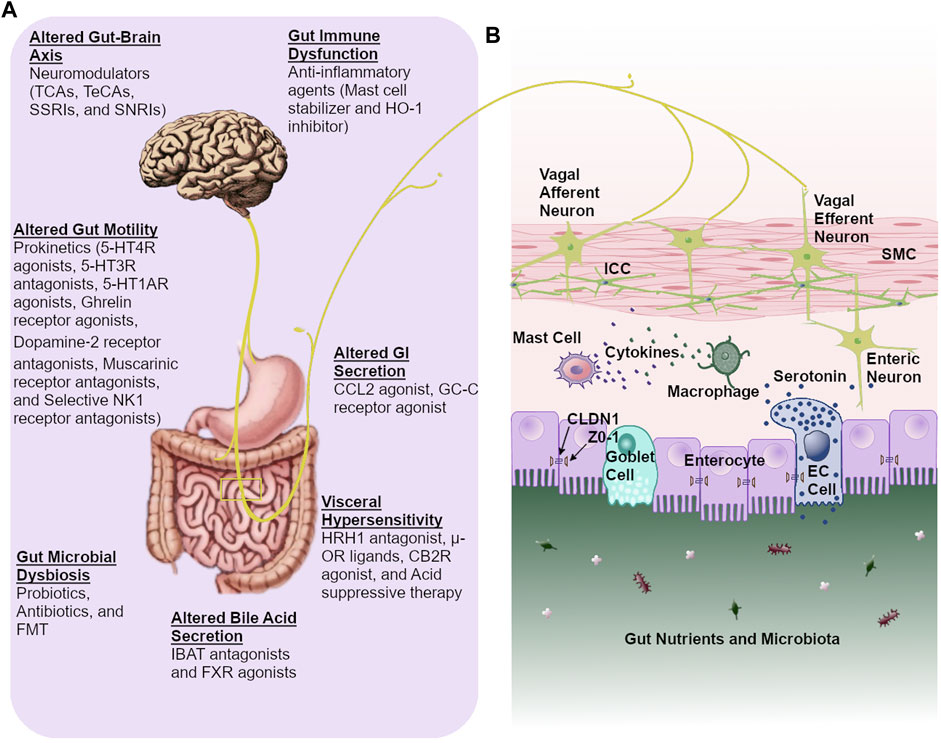
Pharmacological agents currently in clinical trials for disorders in neurogastroenterology. - Abstract - Europe PMC

Clinical trial: the efficacy and tolerability of velusetrag, a selective 5‐HT4 agonist with high intrinsic activity, in chronic idiopathic constipation – a 4‐week, randomized, double‐blind, placebo‐controlled, dose–response study - Goldberg - 2010 -

PDF) Clinical trial: the efficacy and tolerability of velusetrag, a selective 5-HT4 agonist with high intrinsic activity, in chronic idiopathic constipation - a 4-week, randomized, double-blind, placebo-controlled, dose-response study | M. Goldberg -

Comparison of efficacy of pharmacological treatments for chronic idiopathic constipation: a systematic review and network meta-analysis | Gut

Theravance Biopharma Receives FDA Fast Track Designation for Velusetrag (TD-5108) for Idiopathic and Diabetic Gastroparesis